You can test your tank right away, and then test before a normally scheduled a water change.
One of the reasons we test water parameters, is to determine how ofter water changes should be done....
If the water coming from the tap is pH 7.6, but changes to 7.0 before a normal water change, it may mean that the scheduled water changes need to be doubled to maintain proper 7.6 water, that 7.6 stability, is your gold standard, but its not only about just pH.
If after a water change your nitrate concentration is 5 ppm, but creeps up to 20 ppm before the next scheduled water change,
That means you need to double of triple your water change schedule, and volume changed.
That intitial 5 ppm is your gold standard.
For example, where I collect cichlid, nitrates are a 0.00 ppm, norm, and an average 8.2 pH.

So I consider that, my goal, the standard these cichlids need to live by,
and that is what my tanks average should alway be, to maintain the fishes I catch healthy.
A couple test results of the tanks water below.
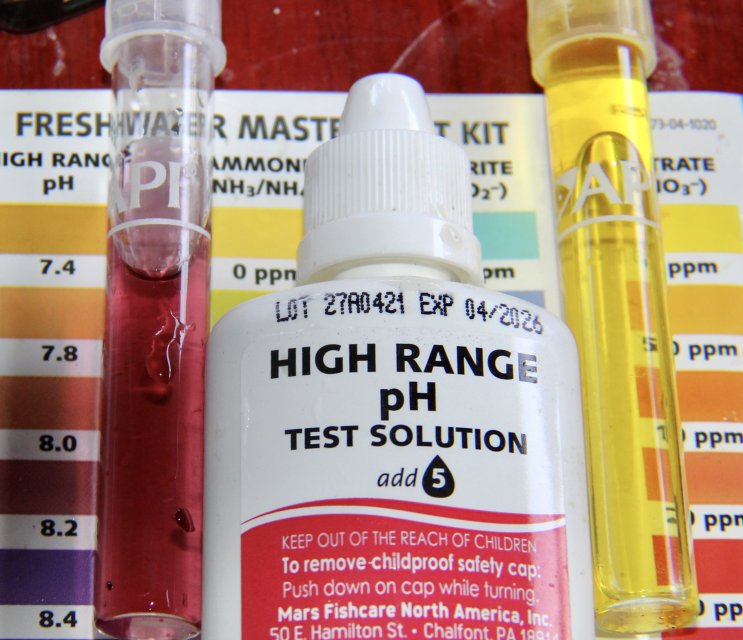


To maintain the standard that meets, healthy conditions where my fish are endemic,
I need to do at least 100% water changes of the tanks water per week ,
and the 180 gallon tank, is also filtered by a 125 gal heavily planted sump.

The planted sump/refufium, above and below.